The carbon intensity of hydrogen production
|
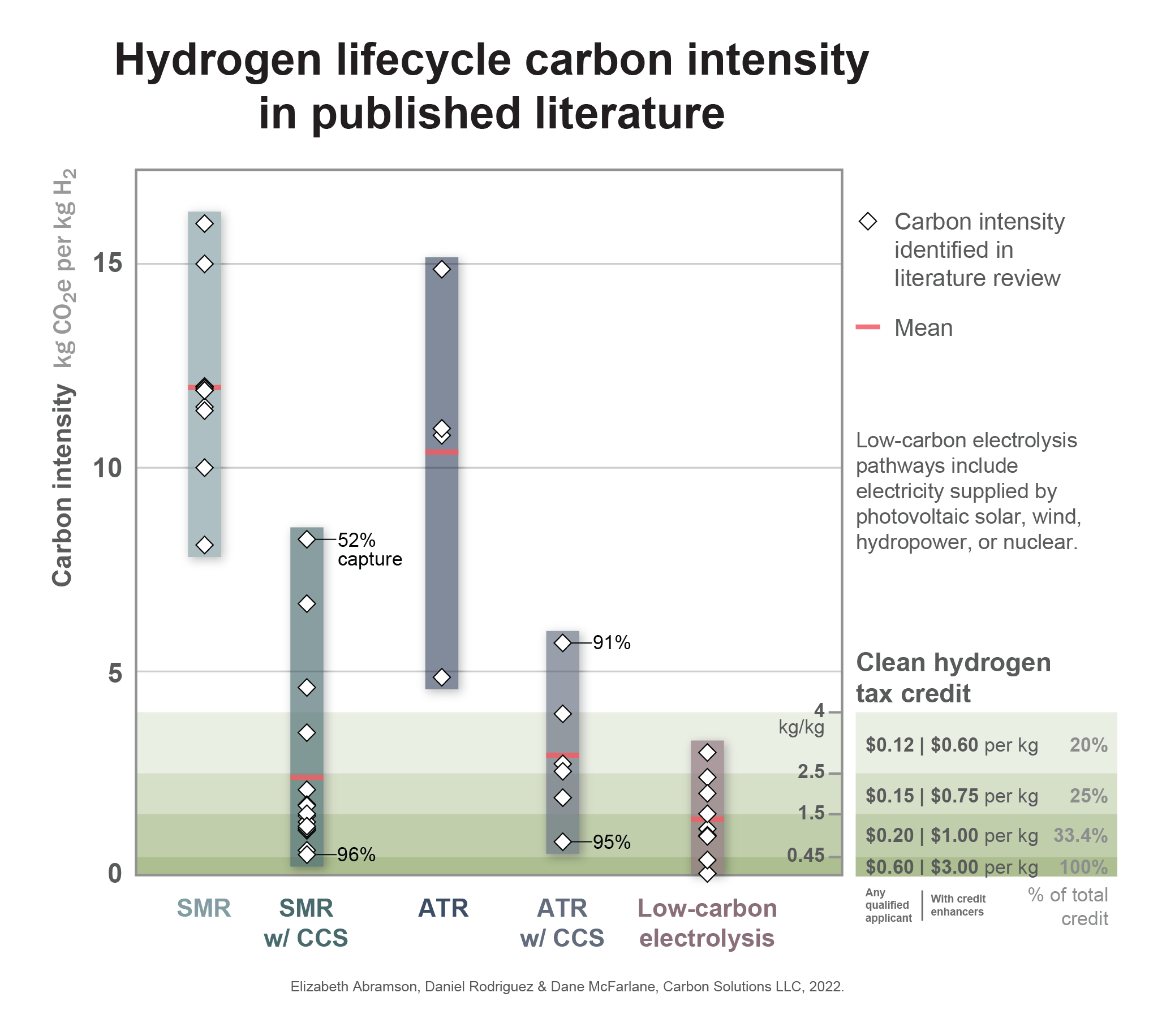
Our recent blog post laid out the relative climate performance of a variety of hydrogen production methods, with each production pathway resulting in unique GHG emissions. Even within a single production pathway, emissions can vary widely depending on plant design, process efficiency, fuel use, and upstream conditions. Our previous blog post highlighted the mean carbon intensities of hydrogen production pathways in published literature and resulted in active discussion and engagement on our posts comparing environmental performance at current real-world facilities to the potential performance of new proposed projects with carbon capture. We also received several inquiries regarding potential eligibility under the hydrogen production tax credit that was included in the recently passed Inflation Reduction Act of 2022. As a result of the engagement with our posts, we created the chart below to give a fuller picture of the range of carbon intensities found in our review of published literature on typical hydrogen production pathways. As we discussed previously, low-carbon hydrogen can be produced through electrolysis powered by low-carbon electricity, or by deploying carbon capture (CCS) on the steam methane reforming (SMR) or autothermal methane reforming (ATR) processes. The carbon intensity of SMR with CCS and ATR with CCS vary across a range of values based on CO2 capture rate, process efficiency levels, energy sources, and upstream conditions. When powered by low-carbon electricity such as from renewable sources, nuclear power, or natural gas with carbon capture, the electrolysis pathway can also achieve very low levels of carbon intensity. Hydrogen production can also involve chemical looping, pyrolysis, biochar, or synthetic fuels, but real-world examples with documented carbon scores for these innovative methods are harder to come by. Any hydrogen facility that achieves a lifecycle carbon intensity below 4 kg CO2e per kg hydrogen is eligible along a sliding scale for the recently passed hydrogen production tax credit. This sliding scale is shown in the lower right of the above chart. Carbon capture considerations for SMR-based and ATR-based hydrogen productionIn published literature and in recent public discourse, including in the engagement and feedback from Carbon Solutions’ recent posts, ATR is often thought to be a more efficient and cost-effective method for hydrogen production than SMR when considering climate constraints and potential for carbon capture. Most literature sources agree that the upper limit for CO2 capture on SMR is around 85-90%, while capture rates can be greater than 90% for ATR, with some sources proposing that it’s feasible for ATR to achieve capture rates greater than 95%. The potential for higher capture rates originates from ATR involving only a single stream of CO2 from syngas purification. While SMR involves syngas purification as well, it also utilizes a preheater or furnace, generally powered by natural gas combustion. The relatively dilute CO2 concentration of natural gas heater emissions results in lower efficiency carbon capture. ATR’s higher capture potential is also due to its injection of pure oxygen into its the reforming reactor, while SMR’s use of ambient air in the reformer results in a lower molar concentration of CO2, reducing the effectiveness of carbon capture. While ATR generally has a higher capture rate than SMR, this does not guarantee a lower carbon intensity due to a variety of factors. ATR operates at much higher temperatures and pressure, requires larger quantities of methane feedstock, and significant electrical energy. One publication in our literature study calculated that ATR required almost three times more electricity than SMR, resulting in a higher carbon intensity for ATR, despite a lower level of underlying process emissions. This means that in the near term, while its process emissions are lower and potential capture rates are higher than SMR, ATR remains a more energy intensive process. Due to its reliance on external electricity, ATR’s carbon intensity can improve as the electric grid decarbonizes, through the use of renewable generation, or through power purchasing agreements. While real-world facilities will vary in their specific configuration, the following process diagrams depict the primary components, emissions sources, and points of CO2 capture found in our literature review of SMR, ATR, and electrolysis-based hydrogen production: Carbon Solutions is embarking on a number of initiatives to support the development of hydrogen hubs throughout the United States. If you are interested in learning more, feel free to get in touch with me at dane.mcfarlane@carbonsolutionsllc.com ContributorsWriter & Editor Lifecycle Assessment & Literature Review Design and Visualization SourcesHydrogen Council, Hydrogen decarbonization pathways: A life-cycle assessment. Hydrogen Council (2021). https://hydrogencouncil.com/wp-content/uploads/2021/04/Hydrogen-Council-Report_Decarbonization_Pathways_Part-1-Lifecycle-Assessment.pdf Collodi G et al, Techno-economic evaluation of deploying CCS in SMR based merchant H2 production with NG as feedstock and fuel. Energy Procedia; 114:2690–712. (2017) https://doi.org/10.1016/j.egypro.2017.03.1533 Oni A et al, Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions, Energy Conversion and Management (2022). https://doi.org/10.1016/j.econman.2022.115245 de Castro J et al, Hydrogen production from natural gas: Auto-thermal reforming and CO2 Capture. Chemical Engineering Transactions; 21:163-168. (2010), https://doi.org/10.3303/CET1021028 Soltani R et al, Assessment of CO2 capture options from various points in steam methane reforming for hydrogen production, International Journal of Hydrogen Energy (2014), http://dx.doi.org/10.1016/j.ihydene.2014.09.161 van Cappellen L et al, Feasibility study into blue hydrogen: Technical, economic & sustainability analysis, CE Delft (2018). https://cedelft.eu/wp-content/uploads/sites/2/2021/04/CE_Delft_9901_Feasibility_study_into_blue_hydrogen_DEF_bak.pdf Ajanovic A et al., The economics and the environmental benignity of different colors of hydrogen. International Journal of Hydrogen Energy (2022). https://doi.org/10.1016/j.ijhydene.2022.02.094 Bhandari R et al., Life cycle assessment of hydrogen production via electrolysis – a review. Journal of Cleaner Production (2014). http://dx.doi.org/10.1016/j.jclepro.2013.07.048 van Cappellen L et al, Feasibility study into blue hydrogen: Technical, economic & sustainability analysis, CE Delft (2018). https://cedelft.eu/wp-content/uploads/sites/2/2021/04/CE_Delft_9901_Feasibility_study_into_blue_hydrogen_DEF_bak.pdf Cetinyaka E et al., Life cycle assessment of various hydrogen production methods. International Journal of Hydrogen Energy (2012). https://doi.org/10.1016/j.ijhydene.2011.10.064 Dufour J et al., Life cycle assessment of alternatives for hydrogen production from renewable and fossil sources. International Journal of Hydrogen Energy (2012). https://doi.org/10.1016/j.ijhydene.2011.09.135 Hamje H et al., Well-to-wheels report version 4.a : JEC well-to-wheels analysis : well-to-wheels analysis of future automotive fuels and powertrains in the European context, Institute for Energy and Transport Joint Research Comissions (2014). https://doi.org/10.2790/95629 Lewis E et al, Comparison of Commercial State-of-the-art, Fossil-Based Hydrogen Production Technologies, National Energy Technology Laboratory (2022). https://doi.org/10.2172/1862910 Pettersen J et al., Blue hydrogen must be done properly. Energy Science & Engineering (2022). https://doi.org/10.1002/ese3.1232 Oni A et al. Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions, Energy Conversion and Management (2022). https://doi.org/10.1016/j.econman.2022.115245 Rostrup-Nielsen J R & Rostrup-Neilsen T, Large scale hydrogen production. CATTECH (2002). https://doi.org/10.1023/A:1020163012266 Salkuyeh Y K et al, Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. International Journal of Hydrogen Energy (2017). http://dx.doi.org/10.1016/j.ijhydene.2017.05.219 Siddiqui O & Dincer I, A well to pump life cycle environmental impact assessment of some hydrogen production routes, International Journal of Hydrogen Energy (2019). https://doi.org/10.1016/j.ijhydene.2019.01.118 Spath P & Mann M, Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming. National Renewable Energy Laboratory (2000). https://doi.org/10.2172/764485 Spath P & Mann M, Life Cycle Assessment of Renewable Hydrogen via Wind/Electrolysis. National Renewable Energy Technology (2004). https://www.osti.gov/servlets/purl/15006927 Suleman F et al., Environmental impact assessment and comparison of some hydrogen production options. International Journal of Hydrogen Energy (2015). http://dx.doi.org/10.1016/j.ijhydene.2015.03.123 |